Abstract
Introduction Hodgkin and non-Hodgkin B-lymphomas are clonal neoplasm of mature B-cells. It has been increasingly recognized that tumor microenvironment (TME) plays a significant role in the initiation, maintenance, progression and immune escape in lymphomagenesis. However, there are limited studies focusing on comprehensive characterization of TME, that could potentially lead to identification of targetable vulnerabilities. Flow cytometry provides a robust approach for single-cell analysis. Nonetheless lack of well-defined comparator groups and/or a robust, reproducible approaches for analyzing the multidimensional data often limited such studies. In the current study we analyzed 251 B-cell lymphoma specimens (Table 1), comparing the tumor infiltrating T cell subsets with a well characterized cohort of reactive lymph nodes (41 follicular hyperplasia; 32 mixed reactive; 3 progressive transformation of germinal centers, PTGC).
Methods On behalf of the imCORE Network, utilizing 2 standardized flow cytometry panels (23 antigen/18-color), we identify the main T-cell subpopulations (CD4, CD8 and gd T-cells), their activation state (Naïve, Central Memory, Effector Memory, Effector) and the immune checkpoint expression (TIGIT, TIM3, PD-1, CD96, LAG3, CTLA4, CD73). Using standardized instrument settings and an R-based algorithms (gaussNorm) to minimize technical variations in sample acquisition we analyzed the T-cell subpopulations using a dimensionality reduction technique (UMAP), combined with an unsupervised clustering algorithm (FlowSOM). Non-parametric test and agglomerative hierarchical clustering have been used for the analysis.
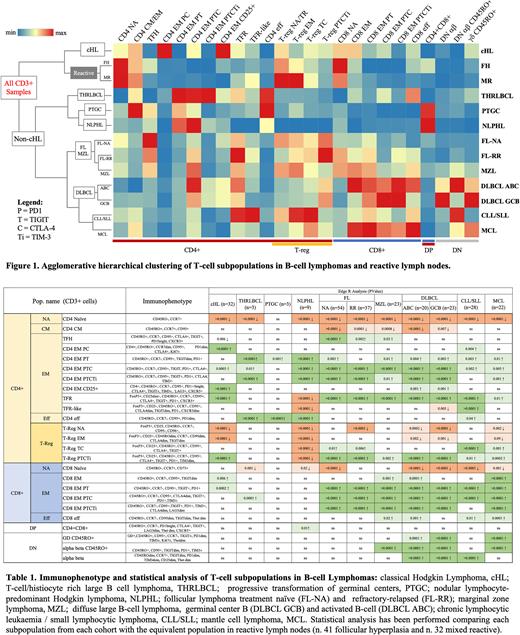
Results While reactive LNs shared a similar T-cell phenotype mainly enriched in naïve cells, most of the B cell lymphoma subtypes were characterized by an immune suppressive microenvironment enriched in specific subsets of activated T regulatory cells (Treg) and/or exhausted memory cells (Figure 1, Table 1). Each histology was characterized but a distinct T-cell profile suggesting unique immune microenvironment for each lymphoma type. Follicular lymphoma (FL) samples were enriched in activated Treg expressing TIGIT, CTLA4 (Treg TC) alone or in combination with and PD1 and TIM3 (Treg PTCTi). T follicular helper cells (TFH), follicular regulatory cells (TFR) and exhausted memory cells expressing CTLA4, TIGIT, PD1 and TIM3 (predominantly in the CD8 compartment) were also expanded in FL cases. In refractory/relapsed FL cases a further expansion of TFR was observed. TFR were also enriched in marginal zone lymphoma (MZL) and small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL) samples, together with T-reg TC in CLL/SLL and Treg PTCTi in MZL. Exhausted T-cells expressing PD1, TIGIT, CTLA-4 and TIM3 were increased in both diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) samples, but only in DLBCL samples CD4 EM expressing CD25, PD1 and CTLA4 (CD4 EM CD25+) are expanded. On the other hand, classic Hodgkin lymphoma was the only B-cell lineage lymphoma characterized by the expansion of memory CD4 expressing dim PD1 and CTLA4 (CD4 EM PC), along with low levels of exhausted CD8+ T cells. In contrast, nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) was the only B cell lymphoma showing almost complete depletion of FoxP3 expressing Tregs, together with an expansion of double positive CD4+CD8+ T cells. Furthermore, it did not reveal an increase in exhausted CD8+ T-cells.
Conclusions Our results suggest that each B-cell lymphoma is characterized by a specific T-cell immune signature. Most subtypes show enrichment in highly suppressive inhibitory cells (Treg and TFR) and exhausted memory T-cells differentially expressing PD1, CTLA4, TIGIT and TIM3. The expression of these inhibitory receptors also reveals a different involvement of the CD4 and CD8 compartments among B-cell lymphomas. Knowledge of these T-cell immune signatures can provide an avenue for identification of novel therapeutic approaches and guide lymphoma specific immunotherapy.
Disclosures
Dogan:Physicians' Education Resource: Consultancy, Honoraria; Loxo: Consultancy; Roche: Other: Research Funding; EUSA Pharma: Consultancy; Seattle Genetics: Consultancy; Peer View: Honoraria; Incyte: Consultancy; Takeda: Other: Research Funding. Zhu:Leica Biosystems: Consultancy. Roshal:Roche: Other: Funding; NGM: Other: Funding; Beat AML: Other: Funding; Auron Therapeutics: Other: Ownership / Equity interests; Provision of services; Celgene: Other: Provision of services; Physicians' Education Resource: Other: Provision of services. Galera:PAIGE.AI: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal